Hours of Operation
Phone Numbers
- Main Number: 312.926.2210
Overview
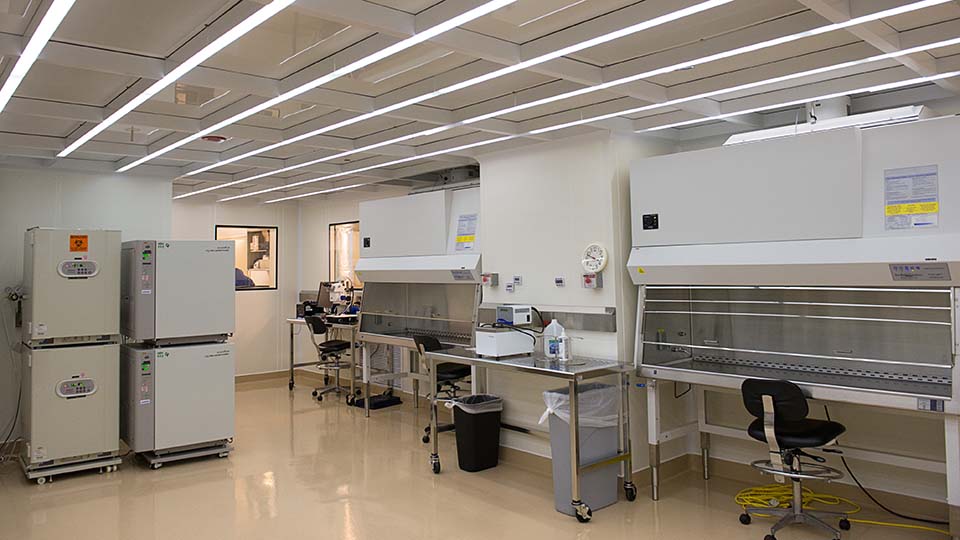
The Mathews Center for Cellular Therapy (MCCT) offers researchers, clinicians and biotech companies regulatory compliant labs and services to manufacture minimally and more than minimally manipulated and complex biotechnology products. Offering:
Four Product Manufacturing Suites
- Good manufacturing practices (GMP)
- Good tissue practices (GTP)
- Adaptable to most biologic and cellular processes for phase I – III clinical trials
Large General Lab
- GTP
- Good laboratory practices (GLP)
- Accommodates minimally and more than minimally manipulated products
More
- Access to 24/7 monitored cryopreservation storage facilities for manufactured products
- CLIA certified testing for release criteria
- Regulatory and process support
You may contact Fenlu Zhu, Ph.D., Director of MCCT at fenlu.zhu@nm.org with any questions or requests.
Parking and Transportation
Parking is available at discounted rates for patients and visitors of Northwestern Memorial Hospital. Download Parking Map
Parking Rates
Standard Parking
Up to 7 Hours: $26 7 to 24 Hours: $12
Parking Validation
Parking garage tickets must be validated each time a car is parked. Without validation, regular garage rates will apply.
Why Choose Us
Northwestern Medicine is proud to have five hospitals ranked among “America’s Best” by U.S. News & World Report for 2023-24. Anchored by Northwestern Memorial Hospital, the only Illinois hospital on the national Honor Roll for 12 straight years, our clinical and administrative staff and medical and science faculty come together every day with a shared commitment to superior quality, academic excellence, scientific discovery and patient safety.
With Northwestern Memorial HealthCare, we’re dedicated to consistently providing high-quality, cost-effective, patient-focused care. We seek to improve the health of the communities, individuals, family and friends we serve by delivering a broad range of services with leading medicine and compassion. Because what makes us better, makes you better.
